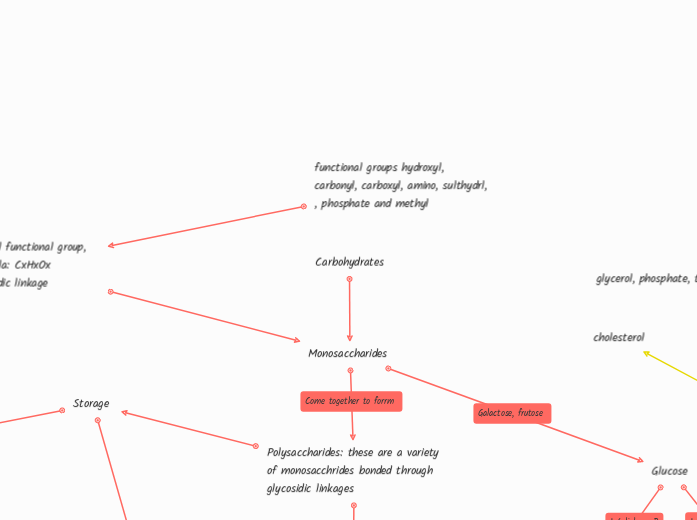
functional groups hydroxyl, carbonyl, carboxyl, amino, sulthydrl, , phosphate and methyl
Carboxl, hydroxl functional group, chemical formula: CxHxOx Link by glycosidic linkage
Monosaccharides
Polysaccharides: these are a variety of monosacchrides bonded through glycosidic linkages
Structure
Cellulose
Beta glucose, 1-4 beta glycosidic linkage, no branching, Plant cell
Storage
Starch: plants
Amylose
No branching, Alpha glucose, 1-4 glycosidic linkage, cannot be easily broken
Helical shaped
Amylopectin
Branching, Alpha glucose, 1-4 glycosidic linkage, can be easily broken
Glycogen: animals
Alpha Glucose, 1-4 glycosidic linkage, branching, easy to break
1-6 Glycosidic linkage
Glucose
Alpha Glucose
Can be broken down by enzymes
Beta Glucose
Cannot be broken down by enzymes
Same chemical formula, different structure arrangement of OH
Chemical bonds: An attraction between two or more atoms, and is what forms a chemical
Hydrogen bonds
covalent bonds
bonds are being equally shared between atoms
Ionic Bonds
Van der Waals
Molecules: A group of atoms connected by bonds
Nonpolar molecules: When atoms bond together to form molecules, they share or give electrons. If the electrons are shared equally by the atoms, then there is no resulting charge,
Polar molecules: Is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar amino acids
Unit 1
glycerol molecule+ fatty acid
triaglycerol
saturate fat molecule
unsaturated fat molecule
cis bond
trans bond
isomers
structural
geometric
enantiomer
Hydrophobic interaction
condensation reaction
A peptide is formed from condensation.
Protein folding
Primary structure- The main chain or amino acids are interacting with peptide bonds. A straight line.
Secondary structure- Folding is beginning to happen it can either be alpha helix or beta pleated sheet. The folding depends on the sequence of the amino acids. Both of these are due to the hydrogen bonds.
Tertiary groups- The folding continues and is folding the secondary structures. Interactions begin between the r groups. The r groups can vary and depending on the r groups depends on the folding of the protein.
The R-group interactions are hydrophobic, hydrophillic, disulfide, van der waals, ionic, and hydrogen bonds.
If there are hydrophobic interactions the protein would fold inwards letting the hydrophobic interact on the inside with other hydrophobic r groups. The hydrophilic interactions would be outwards interacting with other hydrophilic r groups.
Quaternary structure- There are more than 2 polypeptides folded together. Interactions are by the R groups
Protein structure: It is determined by amino acid sequence, physical, and chemical conditions in the proteins environment.
Heating a protein up will denature (unfold) the protein to a straight line.
single bond
double bond
esther bond
phospholipid
Lipid
phospholipid
cholesterol
amphithatic molecule
glycerol, phosphate, two fatty acids
Nucleic Acids
Polymers made of monomers called nucleotides
Nitrogenous bases
Nucleotide: pentose sugar, nitrogenous base, 1 or 2 phosphate groups
Pyrimidines: Cyosine(C), thymine(T), uracil
Phosphate group
Phosphodiester bond: The bond in which connects the polymer
Nucleoside: Nitrogenous base and pentose sugar
DNA
Deoxyribose sugar
RNA
Ribose sugar
Purines: Guaine (G), Adenine(A)
Carbohydrates
result from the attraction between oppositely charged ions.
Proteins
Main chain
Amino Group(-)
Carboxyl group (+)
central carbon
R-groups (Side chains)
Nonpolar amino acids
Basic amino acids
Positively charged
Acidic amino acids
Negatively Charged